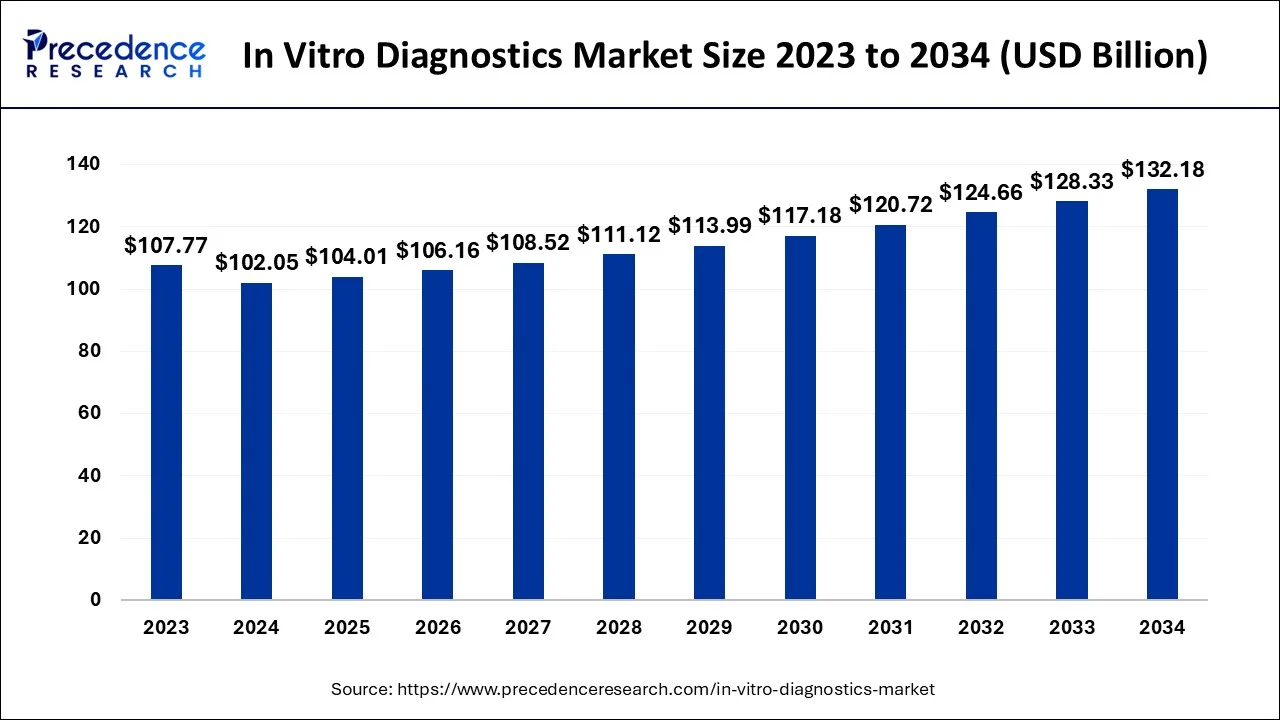
The global in vitro diagnostics (IVD) market size is calculated at USD 102.05 billion in 2024, and is projected to cross around USD 132.18 billion by 2034 with a CAGR of 2.62%.
Get Sample Copy of Report@ https://www.precedenceresearch.com/sample/1130
Key Points
- North America captured 42% of the total market revenue in 2023.
- The reagents segment led the market by product, contributing 66% of the revenue.
- Infectious diseases emerged as the leading application, accounting for 53% of total revenue.
- The laboratory segment recorded a 38% revenue share among end-uses.
- The point-of-care segment held the highest market share in terms of test location.
Global In Vitro Diagnostics Market Revenue, By Region, 2023-2033 (USD Million)
| Region | 2023 | 2024 | 2030 | 2033 |
| North America | 45,485.13 | 42,856.34 | 47,752.84 | 51,537.64 |
| Europe | 28,504.19 | 26,937.78 | 30,562.63 | 33,275.64 |
| APAC | 25,200.01 | 24,101.53 | 29,377.75 | 33,110.71 |
| LA | 4,953.16 | 4,709.10 | 5,538.43 | 6,135.84 |
| MEA | 3,624.70 | 3,444.33 | 3,948.70 | 4,267.14 |
Market Scope
| Report Coverage | Details |
| Market Size in 2024 | USD 102.05 Billion |
| Market Size by 2034 | USD 132.18 Billion |
| Growth Rate from 2024 to 2034 | CAGR of 2.62% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2034 |
| Segments Covered | Product, Technology, Application |
| Regional Scope | North America, APAC, Europe, Latin America, MEAN, Rest of the World |
Read Also:http://www.expresswebwire.com/medical-devices-market/
Market Dynamics
Market Drivers
The rising burden of infectious diseases, including COVID-19, tuberculosis, and HIV, has accelerated the demand for in vitro diagnostics. Increasing government funding for healthcare and research initiatives is fueling market expansion. Technological innovations, such as portable diagnostic devices and lab-on-a-chip technologies, are improving diagnostic accuracy and efficiency.
Additionally, growing collaborations between pharmaceutical companies and diagnostic manufacturers are enhancing the development of novel diagnostic solutions.
Opportunities
The increasing adoption of personalized medicine and companion diagnostics presents a major growth opportunity in the IVD market. The shift towards non-invasive and minimally invasive diagnostic techniques is gaining traction, enhancing patient compliance.
Emerging economies with expanding healthcare access and government support for diagnostic testing offer untapped potential. Additionally, automation and artificial intelligence-driven diagnostic solutions are expected to enhance test accuracy and efficiency, opening new avenues for market expansion.
Challenges
A major challenge in the IVD market is the lack of standardization in testing procedures, leading to variations in test accuracy and reliability. Reimbursement policies for diagnostic tests vary across regions, making it difficult for companies to establish consistent pricing strategies.
The high cost of molecular and genetic testing can be a barrier to widespread adoption. Moreover, increasing regulatory scrutiny and data privacy concerns related to digital diagnostics are creating additional hurdles for manufacturers.
Regional Insights
North America continues to lead the IVD market, supported by advanced healthcare infrastructure, strong research investments, and favorable regulatory policies. Europe is witnessing steady growth due to rising healthcare expenditures and increased adoption of molecular diagnostics.
The Asia-Pacific region is experiencing rapid expansion, driven by increasing government healthcare initiatives, improving laboratory infrastructure, and rising demand for infectious disease diagnostics. Latin America and the Middle East & Africa are gradually advancing in the IVD sector, but challenges such as affordability and accessibility still hinder growth.
In Vitro Diagnostics Market Companies
Existence of research organizations that offer clinical research, consulting services, and laboratory testing to cater the swelling demand for rapid diagnosis and treatment plans is likely to fuel the IVD market.
The key players focus on strategies such as acquisitions, mergers, collaboration sand development of technologically advanced products in order to gain the competitive edge in the market. The major key players in the in vitro diagnostics market include:
- Alere, Inc.
- Hoffmann-La Roche Ltd.
- Arkray
- Beckman Coulter
- Becton Disckinson
- Bio-Rad laboratories
- Danaher
- Sysmex Corporation
- Abbott Laboratories
Segments Covered in the Report
This research study comprises complete assessment of the market by means of far-reaching qualitative and quantitative perceptions, and predictions regarding the market. This report delivers classification of marketplace into impending and niche sectors.
Further, this research study calculates market size and its development drift at global, regional, and country from 2023 to 2033. This report contains market breakdown and its revenue estimation by classifying it based on product, application, technology and region as follows:
By Product
- Reagents
- Instruments
- Services
By Test Location
- Point of Care
- Home Care
- Others
By Technology
- Immunoassay
- Instruments
- Reagents
- Services
- Hematology
- Instruments
- Reagents
- Services
- Clinical Chemistry
- Instruments
- Reagents
- Services
- Molecular Diagnostics
- Instruments
- Reagents
- Services
- Coagulation
- Instruments
- Reagents
- Services
- Microbiology
- Instruments
- Reagents
- Services
- Others
- Instruments
- Reagents
- Services
By Application
- Diabetes
- Cardiology
- Nephrology
- Infectious Disease
- Oncology
- Drug Testing
- Autoimmune Diseases
- Others
By End User
- Standalone Laboratories
- Hospitals
- Academic & Medical Schools
- Point-of-Care
- Others
By Regional
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Middle East & Africa
- Latin America
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/
