The global tubeless insulin pump market size reached USD 2.05 billion in 2023 and is projected to hit around USD 11.93 billion by 2033, growing at a CAGR of 19.25% from 2024 to 2033.
Key Points
- North America held the largest market share of 55% in 2023.
- Asia Pacific is expected to grow at the fastest rate during the forecast period.
- By type, the insulin patch pump segment accounted for the dominating share in 2023. The segment is observed to continue growth at a significant rate in the upcoming period.
- By component, the pod or patch segment held the largest share of the market in 2023.
- By end users, the hospitals segment held the largest share of the market in 2023.
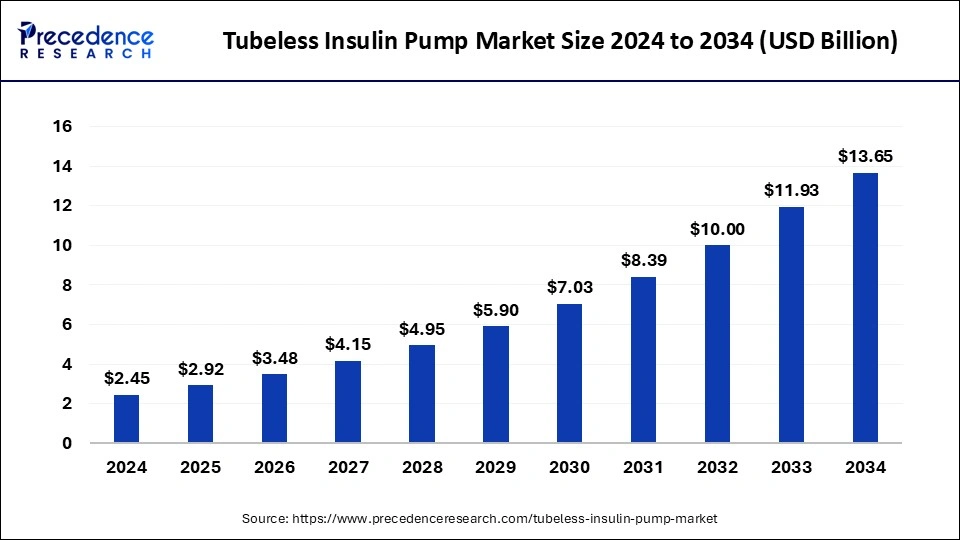
The tubeless insulin pump market is witnessing substantial growth globally, driven by increasing prevalence of diabetes and the demand for advanced insulin delivery systems. Tubeless insulin pumps are innovative devices designed to provide continuous subcutaneous insulin infusion without the need for tubing. This technology offers greater convenience, flexibility, and discretion to individuals managing diabetes, leading to a rising adoption rate. As the market continues to evolve, manufacturers are focusing on enhancing product features, improving user experience, and expanding their distribution networks to capitalize on the growing demand.
Get a Sample:https://www.precedenceresearch.com/sample/3944
Table of Contents
ToggleGrowth Factors
Several factors contribute to the growth of the tubeless insulin pump market. Firstly, the increasing incidence of diabetes worldwide is a significant driver. With the prevalence of both type 1 and type 2 diabetes on the rise, there is a growing need for effective insulin delivery systems that can help patients manage their condition more efficiently. Tubeless insulin pumps offer advantages such as precise insulin delivery, reduced risk of infusion site issues, and improved quality of life, driving their adoption among diabetic patients.
Secondly, technological advancements play a crucial role in fueling market growth. Manufacturers are constantly innovating to develop tubeless insulin pumps with advanced features such as automated insulin delivery, continuous glucose monitoring integration, and smartphone connectivity. These innovations not only enhance the convenience and effectiveness of insulin therapy but also cater to the preferences of tech-savvy consumers.
Moreover, the shift towards personalized healthcare and patient-centric treatment approaches is boosting the demand for customized insulin delivery solutions. Tubeless insulin pumps allow for individualized insulin dosing regimens and flexibility in lifestyle management, making them increasingly attractive to patients seeking tailored treatment options.
Additionally, the expanding geriatric population, coupled with the growing awareness about the benefits of insulin pump therapy, is driving market growth. Elderly individuals with diabetes often face challenges associated with insulin administration, such as dexterity issues and cognitive decline. Tubeless insulin pumps offer a user-friendly alternative to traditional insulin injections, making them particularly appealing to older patients.
Furthermore, favorable reimbursement policies and government initiatives aimed at improving diabetes management are supporting market expansion. Reimbursement coverage for tubeless insulin pumps encourages greater adoption among patients, particularly in regions with high healthcare expenditure and a strong focus on chronic disease management.
Tubeless Insulin Pump Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 19.25% |
| Global Market Size in 2023 | USD 2.05 Billion |
| Global Market Size by 2033 | USD 11.93 Billion |
| U.S. Market Size in 2023 | USD 790 Million |
| U.S. Market Size by 2033 | USD 4,590 Million |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Type, By Component, and By End-users |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Tubeless Insulin Pump Market Dynamics
Drivers:
Several drivers are propelling the growth of the tubeless insulin pump market. Firstly, the increasing prevalence of diabetes worldwide is a key driver. With the global diabetes epidemic showing no signs of abating, there is a growing need for innovative insulin delivery systems that can help patients manage their condition effectively. Tubeless insulin pumps offer advantages such as precise insulin delivery, flexibility in dosing, and reduced risk of hypoglycemia, making them an attractive option for diabetic individuals seeking improved quality of life.
Secondly, technological advancements in insulin pump technology are driving market growth. Manufacturers are constantly innovating to develop tubeless insulin pumps with advanced features such as automated insulin delivery algorithms, continuous glucose monitoring integration, and smartphone connectivity. These innovations not only enhance the convenience and effectiveness of insulin therapy but also cater to the preferences of tech-savvy consumers who seek seamless connectivity and remote monitoring capabilities.
Moreover, the increasing adoption of wearable medical devices is fueling demand for tubeless insulin pumps. As wearable technology becomes more ubiquitous, patients are increasingly turning to tubeless insulin pumps as a convenient and discreet option for insulin delivery. The compact size, lightweight design, and waterproof capabilities of tubeless insulin pumps make them ideal for continuous wear, allowing patients to maintain their insulin therapy regimen while engaging in various activities and daily routines.
Additionally, the growing emphasis on personalized medicine and patient-centric care is driving market growth. Tubeless insulin pumps offer greater flexibility in insulin dosing regimens and lifestyle management, allowing for individualized treatment approaches tailored to each patient’s unique needs and preferences. This customization not only improves patient satisfaction and adherence but also enhances clinical outcomes by optimizing glycemic control and minimizing the risk of complications.
Furthermore, favorable reimbursement policies and healthcare reforms aimed at improving access to diabetes management tools are supporting market expansion. In many countries, reimbursement coverage for tubeless insulin pumps has increased, making them more affordable and accessible to patients, particularly those from underserved populations or with limited financial resources.
Restraints:
Despite the promising growth prospects, the tubeless insulin pump market faces certain restraints that may hinder its expansion. One significant challenge is the high cost associated with tubeless insulin pump therapy. While these devices offer numerous benefits compared to traditional insulin injections, they are often more expensive upfront and may incur ongoing expenses for supplies and maintenance. This cost barrier can limit access to tubeless insulin pumps for patients with limited financial means or inadequate insurance coverage, thereby restricting market growth.
Another restraint is the complexity of transitioning to tubeless insulin pump therapy for some patients. While tubeless insulin pumps offer greater convenience and flexibility, they require a learning curve for proper use and management. Patients must undergo training and education to become proficient in operating the device, adjusting insulin doses, and troubleshooting technical issues. This transition process can be daunting for individuals accustomed to conventional insulin injection therapy, leading to reluctance or resistance to adopt tubeless insulin pumps.
Additionally, concerns about the reliability and accuracy of tubeless insulin pump technology may impede market growth. Although modern insulin pumps are equipped with advanced features for precise insulin delivery and continuous glucose monitoring, they are not immune to technical malfunctions or errors. Patients and healthcare providers may harbor doubts or skepticism about the safety and efficacy of tubeless insulin pumps, particularly in critical situations such as hypoglycemia or hyperglycemia emergencies. Addressing these concerns through robust clinical evidence, real-world data, and regulatory oversight is essential to instill confidence and trust in tubeless insulin pump therapy.
Moreover, regulatory hurdles and compliance requirements pose challenges for manufacturers seeking to introduce new tubeless insulin pump products or expand into different geographic markets. Obtaining regulatory approvals and navigating the complex regulatory landscape can be time-consuming and resource-intensive, delaying product launches and market entry strategies. Furthermore, varying reimbursement policies and healthcare regulations across different countries or regions may create barriers to market access and adoption, requiring tailored approaches and strategic partnerships to overcome.
Opportunity:
Despite the challenges, the tubeless insulin pump market presents significant opportunities for growth and innovation. One key opportunity lies in expanding market penetration among underserved patient populations, including children, adolescents, and elderly individuals with diabetes. Tailoring tubeless insulin pump designs, features, and educational resources to meet the unique needs and preferences of these demographic segments can help drive adoption and improve clinical outcomes.
Furthermore, leveraging digital health technologies and data analytics offers opportunities to enhance the functionality and connectivity of tubeless insulin pumps. Integrating artificial intelligence algorithms, machine learning models, and predictive analytics into insulin pump systems can enable personalized insulin dosing recommendations, proactive glucose management, and remote monitoring capabilities. This convergence of technology and healthcare holds promise for optimizing diabetes management and empowering patients to take control of their health.
Moreover, partnerships and collaborations between industry stakeholders, including manufacturers, healthcare providers, payers, and patient advocacy organizations, can unlock new opportunities for innovation and market expansion. By pooling resources, expertise, and insights, these partnerships can accelerate the development and adoption of tubeless insulin pump solutions, address unmet needs, and drive positive change in diabetes care delivery.
Additionally, expanding market reach beyond established regions and into emerging markets presents lucrative opportunities for growth. Rapid urbanization, changing lifestyles, and increasing disposable incomes in emerging economies are driving the demand for advanced healthcare technologies, including tubeless insulin pumps. By tailoring product offerings, pricing strategies, and distribution channels to local market.
Read Also: Protective Face Masks Market Size to Reach USD 16.32 Bn by 2033
Recent Developments
- In August 2023, the Tubeless insulin pump received FDA clearance for diabetes people. The Accu-Chek Solo micropump (Roche Diabetes) was granted 510(k) clearance from the FDA. Accu-Chek Solo micropump is tubeless, small, and lightweight. Users can place the device on four different infusion sites on the body. The device is detachable, allowing people with diabetes to change the infusion site when necessary.
- In July 2023, the FDA announced the clearance of Tandem Diabetes Care’s Mobi durable automated insulin pump. The approval covers people with diabetes aged six and above, expanding Tandem’s portfolio of products. Mobi features a 200-unit insulin cartridge and an on-pump button to provide an alternative to phone control for insulin boluses.
- In February 2024, Omnipod 5 received approval to integrate with the new freestyle libre two plus sensor. The Omnipod 5 hybrid closed loop system has received approval to integrate with the Abbott FreeStyle Libre 2 Plus Sensor in the UK, which will give people living with diabetes additional choice and flexibility for managing their blood sugar levels.
- In April 2023, Insulet announced FDA clearance of Omnipod GO, a first-of-its-kind basal-only insulin pod for people with type 2 diabetes aged 18 and above. Omnipod GO is a wearable, standalone insulin delivery system that provides a fixed rate of continuous rapid-acting insulin for 72 hours.
Tubeless Insulin Pump Market Companies
- Medtronic plc
- Hoffmann-La Roche Ltd
- Tandem Diabetic Care, Inc.
- Insulet Corporation
- Ypsomed
- Cellenovo
- Abbott
- Tandem Diabetes Care
- Insulet Corporation
- Sooil Development
- Valeritas, Inc
- JingasuDelfu Co., Ltd.
- Cellnova
- Roche Holdings
- Spring Health Solutions
- Johnson & Johnson
- Medtrum Technologies
- Debiotech
- CeQur
- Valeritas Holding
- Animas Corporation
Segments Covered in the Report
By Type
- Insulin Patch Pump
- Traditional Pump
By Component
- Pod or Patch
- Remote
- Accessories
By End-users
- Hospitals
- Pharmacies
- E-commerce
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.uswebwire.com/
