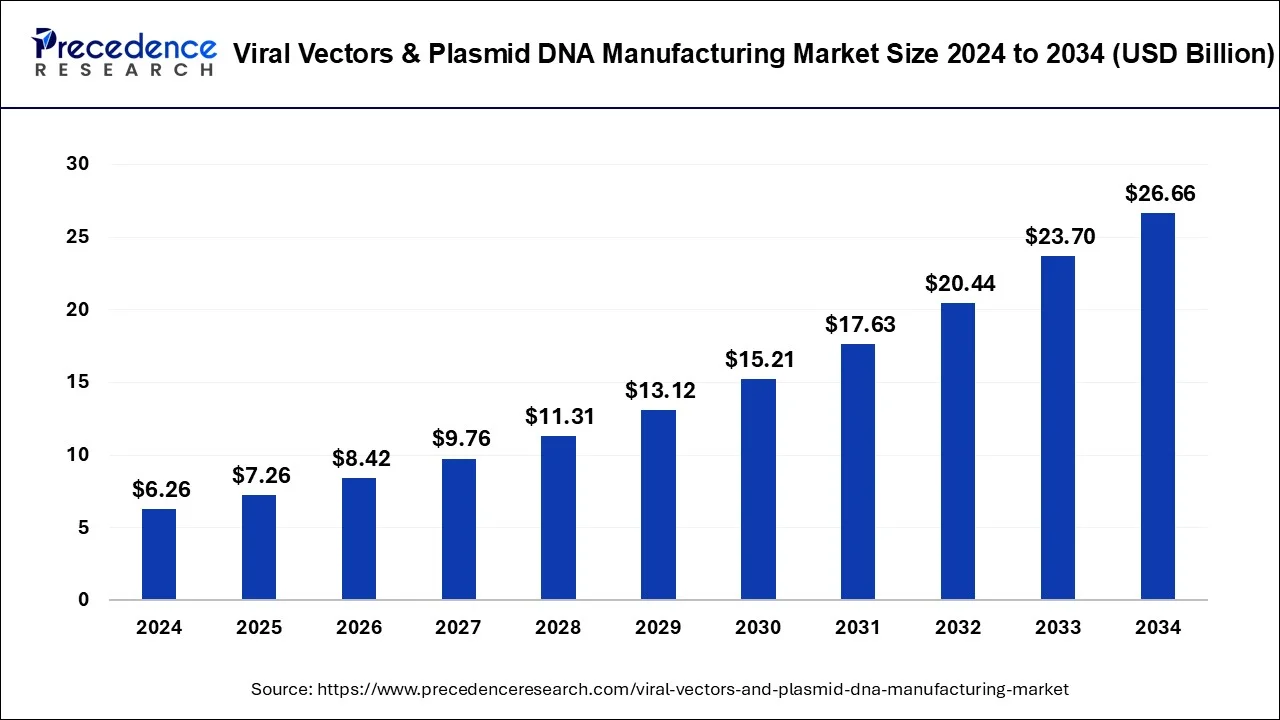
The global viral vectors & plasmid DNA manufacturing market size reached USD 6.26 billion in 2024 and is expected to be attain around USD 26.66 billion by 2034 with a CAGR of 15.59%.
Get Sample Copy of Report@ https://www.precedenceresearch.com/sample/1012
Key Takeaways
- In 2024, North America secured the highest market share, contributing 49% of total revenue.
- The AAV segment led the vector type category, accounting for 21% of the market revenue in 2024.
- The downstream processing segment remained dominant in workflow, representing 54% of the revenue share in 2023.
- The vaccinology segment held the top position in application, generating a revenue share of 22.5% in 2024.
- Cancer remained the leading disease category, capturing 38% of the total revenue in 2024.
- Research institutes emerged as the primary end-use sector, holding approximately 58.4% of the market share in 2024.
Market Overview
The viral vectors and plasmid DNA manufacturing market is witnessing significant growth due to advancements in gene therapy and vaccine development. The demand for efficient genetic material production has surged as biotech companies focus on developing novel therapies for rare and chronic diseases. With continuous innovations, the market is poised for further expansion.
Drivers
The increasing prevalence of genetic disorders and the growing success of gene therapy treatments are key drivers of the market. The rise in demand for cell-based immunotherapies, such as CAR-T cell therapy, has also contributed to market growth. Additionally, the COVID-19 pandemic accelerated investments in viral vector-based vaccine production, boosting overall market development.
Opportunities
Advancements in bioprocessing technologies provide opportunities for large-scale and cost-effective manufacturing. The growing adoption of CRISPR and other gene-editing technologies further expands the market scope. Additionally, government funding and strategic partnerships among biotech firms and research institutions are fostering innovation and expansion in the sector.
Challenges
Manufacturing complexity and high operational costs pose major challenges in scaling production. Regulatory scrutiny is another critical factor, as stringent approval processes can delay the launch of new gene therapies. Furthermore, supply chain limitations and the need for specialized infrastructure add to the industry’s challenges.
Regional Insights
The United States dominates the market due to its strong biotech ecosystem and significant investments in gene therapy research. European countries are also advancing in this sector, particularly in the United Kingdom and Germany. Meanwhile, Asia-Pacific is emerging as a key player, with China and India investing heavily in biotechnology and genetic research.
Viral Vectors & Plasmid DNA Manufacturing Market Companies
- Novasep
- Aldevron
- MerckWaismanBiomanufacturing
- Creative Biogene
- The Cell and Gene Therapy Catapult
- Cobra Biologics
- uniQure N.V.
- Addgene
- FUJIFILM Holdings Corporation
- Oxford Biomedicaplc
- Takara Bio Inc.
Recent Developments
- In May 2024, Charles River Laboratories International, Inc. declared the starting of its Modular and Fast Track viral vector technology transfer frameworks.
- In November 2023, SK Pharmteco Co., South Korean conglomerate SK Group’s contract development and manufacturing organization, has recently started two new viral vector platforms expected to save time and cost in manufacturing innovative cell and gene therapies (CGTs).
- In February 2023, ProBio and RVAC Medicines Pte. Ltd. declared an agreement to establish a strategic partnership to produce GMP-grade plasmid DNA (pDNA) for its mRNA COVID-19 vaccine candidate, RVM-V001, along with collaboration for future therapeutic pipelines.
Market Segmentation
This research report estimates revenue growth at global, regional, and country levels and offers an analysis of present industry trends in everysub-segment from 2025 to 2034. This research study analyzes market thoroughly by classifying global viral vectors & plasmid DNA manufacturing market report on the basis of different parameters including type of vector, application, workflow, end users, disease, and region:
By Vector Type
- Adenovirus
- Plasmid DNA
- Lentivirus
- Retrovirus
- AAV
- Others
By Application
- Gene Therapy
- Antisense &RNAi
- Cell Therapy
- Vaccinology
By Workflow
- Upstream Processing
- Vector Recovery/Harvesting
- Vector Amplification & Expansion
- Downstream Processing
- Fill-finish
- Purification
By End-User
- Biopharmaceutical and Pharmaceutical Companies
- Research Institutes
By Disease
- Genetic Disorders
- Cancer
- Infectious Diseases
- Others
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/
