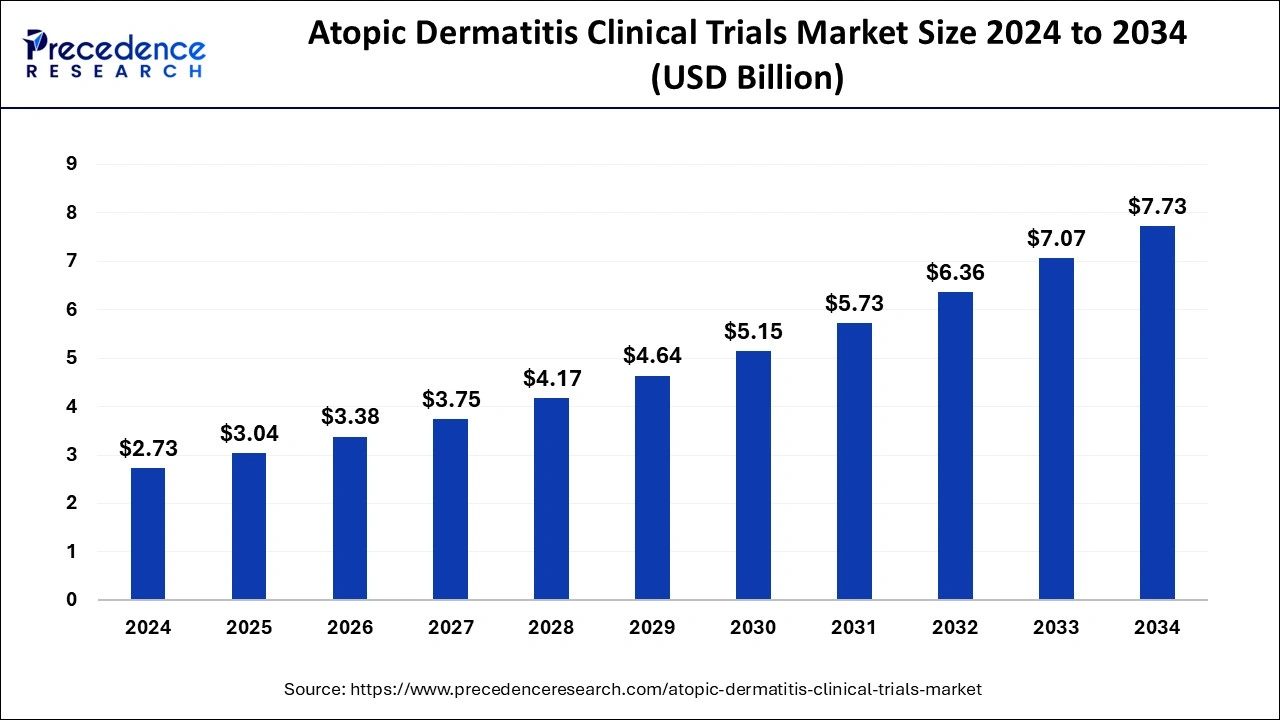
The global atopic dermatitis clinical trials market size reached USD 2.46 billion in 2023 and is projected to hit around USD 7.07 billion by 2033, expanding at a CAGR of 11.14% from 2024 to 2033.
Key Points
- North America has contributed more than 37% of market share in 2023.
- Asia-Pacific is estimated to expand the fastest CAGR between 2024 and 2033.
- By molecule type, the large molecules segment has held the largest market share of 54% in 2023.
- By molecule type, the small molecules segment is anticipated to grow at a remarkable CAGR of 12.5% between 2024 and 2033.
- By study design, the interventional trials segment has generated over 72% of market share in 2023.
- By study design, the observational trials segment is expected to expand at the fastest CAGR over the projected period.
- By phase, the phase II segment has accounted more than over 48% of market share in 2023.
- By phase, the phase III segment is expected to expand at the fastest CAGR over the projected period.
Atopic dermatitis, commonly known as eczema, is a chronic inflammatory skin condition characterized by itchy and inflamed skin. It affects people of all ages, but it is particularly prevalent in children. The condition can have a significant impact on patients’ quality of life, leading to discomfort, sleep disturbances, and psychological stress. Clinical trials play a crucial role in advancing our understanding of atopic dermatitis and developing new treatment options. The Atopic Dermatitis Clinical Trials Market encompasses a wide range of research activities aimed at evaluating the safety and efficacy of various therapies for managing this condition.
Get a Sample: https://www.precedenceresearch.com/sample/4021
Growth Factors
The Atopic Dermatitis Clinical Trials Market is experiencing significant growth due to several key factors. Firstly, the increasing prevalence of atopic dermatitis worldwide has led to a growing demand for novel treatment options. The rising awareness about the impact of the disease on patients’ lives and the need for effective management strategies has spurred investment in clinical research. Additionally, advancements in medical technology and understanding of the underlying mechanisms of atopic dermatitis have facilitated the development of innovative therapies, driving the growth of clinical trials in this field.
Region Insights:
Clinical trials for atopic dermatitis are conducted globally, with participation from research institutions, pharmaceutical companies, and academic centers across different regions. North America and Europe are among the leading regions in terms of the number of clinical trials conducted, owing to the presence of a well-established healthcare infrastructure and a high prevalence of atopic dermatitis in these regions. Asia-Pacific is also emerging as a significant market for atopic dermatitis clinical trials, driven by the increasing prevalence of the disease and the growing focus on research and development in countries like China, Japan, and South Korea.
Atopic Dermatitis Clinical Trials Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 11.14% |
| Global Market Size in 2023 | USD 2.46 Billion |
| Global Market Size by 2033 | USD 7.07 Billion |
| U.S. Market Size in 2023 | USD 640 Million |
| U.S. Market Size by 2033 | USD 1,830 Million |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Molecule Type, By Study Design, and By Phase |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Atopic Dermatitis Clinical Trials Market Market Dynamics
Drivers:
Several factors are driving the growth of the Atopic Dermatitis Clinical Trials Market. One of the primary drivers is the need for effective and safe treatment options for atopic dermatitis patients, particularly those with severe or refractory forms of the disease. Clinical trials provide an opportunity to evaluate the efficacy and safety of novel therapies, including biologics, small molecules, and topical agents. Additionally, regulatory agencies’ increasing emphasis on evidence-based medicine and the requirement for rigorous clinical testing before drug approval have fueled the demand for clinical trials in this field.
Opportunities:
The Atopic Dermatitis Clinical Trials Market presents numerous opportunities for stakeholders involved in research and drug development. With the growing understanding of the pathophysiology of atopic dermatitis, there is potential for the development of targeted therapies that address specific disease mechanisms. Biomarker identification and personalized medicine approaches offer opportunities for tailoring treatment strategies to individual patients’ needs, improving treatment outcomes, and reducing healthcare costs. Furthermore, collaborations between academia, industry, and patient advocacy groups can facilitate the design and execution of innovative clinical trials that address unmet medical needs in atopic dermatitis management.
Challenges:
Despite the promising growth prospects, the Atopic Dermatitis Clinical Trials Market faces several challenges. One of the significant challenges is patient recruitment and retention, particularly for long-term studies involving multiple visits and interventions. Recruitment difficulties can lead to delays in trial timelines and increased costs. Additionally, variability in disease presentation and response to treatment among atopic dermatitis patients can complicate trial design and interpretation of results. Furthermore, regulatory requirements for clinical trials continue to evolve, necessitating careful navigation of complex approval processes and adherence to Good Clinical Practice guidelines.
Read Also: Air Traffic Control (ATC) Equipment Market Size, Growth, Report 2033
Recent Developments
- In October 2023, LEO Pharma disclosed the favorable outcome of the DELTA 3 trial. This Phase 3 extension trial assessed delgocitinib cream, an investigational topical pan-Janus kinase (JAK) inhibitor, for potential treatment in adults with moderate to severe chronic hand eczema (CHE). This positive result bolstered the company’s pipeline value and is expected to facilitate the commercialization of the product.
- In May 2023, Dermavant Sciences, Inc. unveiled encouraging results from ADORING 1, Phase 3 studies investigating the efficacy and safety of topical VTAMA (tapinarof) cream 1% in adults and pediatric subjects as young as 2 years old with moderate to severe atopic dermatitis. These findings provided the company with a route to commercial success, expansion in the market, competitive differentiation, and increased investor support.
Atopic Dermatitis Clinical Trials Market Companies
- Pfizer Inc.
- Sanofi SA
- Regeneron Pharmaceuticals, Inc.
- AbbVie Inc.
- Novartis International AG
- Eli Lilly and Company
- GlaxoSmithKline plc
- Johnson & Johnson
- Leo Pharma A/S
- Dermira, Inc. (acquired by Eli Lilly and Company)
- Galderma S.A.
- AnaptysBio, Inc.
- Dermavant Sciences, Inc.
- LEO Pharma A/S
- Amgen Inc.
Segments Covered in the Report
By Molecule Type
- Small Molecules
- Large Molecules
By Study Design
- Interventional
- Observational
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
