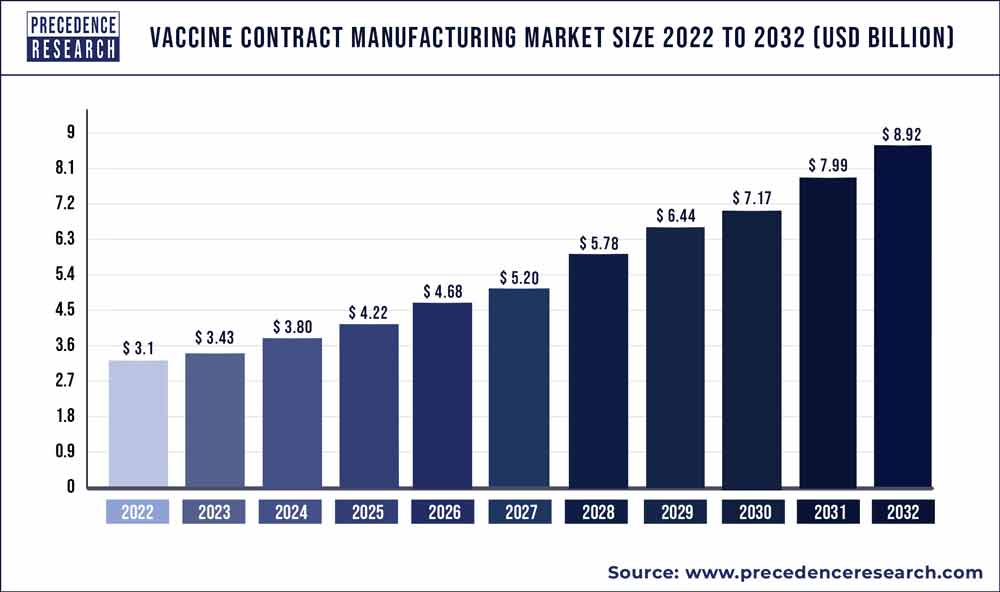
The vaccine contract manufacturing market size is poised to grow by USD 8.92 billion by 2032 from USD 3.1 billion in 2022, exhibiting a CAGR of 11.2% during the forecast period 2023-2032.
Key Takeaways
- North America led the market with the highest market share of 42% in 2022.
- Asia-Pacific is expected to grow at the fastest CAGR during the forecast period.
- By Vaccine Type, the attenuated vaccines segment has held the largest revenue share of 28% in 2022.
- By Vaccine Type, the inactivated inoculations segment is projected to expand at a remarkable CAGR of 12.7% during the projected period.
- By Workflow, the downstream segment generated more than 68% of revenue share in 2022.
- By Workflow, the upstream segment is anticipated to grow at the fastest CAGR over the projected period.
- By Application, the human-use segment held the major revenue share of 69% in 2022.
- By Application, the veterinary segment is estimated to grow at a noteworthy CAGR of 13.3% over the predicted period.
Vaccine Contract Manufacturing Market Overview
The Vaccine Contract Manufacturing Market revolves around the outsourcing of vaccine production to third-party manufacturers. As the global demand for vaccines continues to rise, pharmaceutical companies are increasingly turning to contract manufacturing organizations (CMOs) to meet production needs efficiently. This market segment encompasses a wide range of vaccines, including those for infectious diseases, chronic illnesses, and emerging health threats. The outsourcing of vaccine manufacturing allows companies to leverage the expertise and infrastructure of CMOs, ultimately facilitating the timely and cost-effective production of vaccines to address public health needs.
Get a Sample Report: https://www.precedenceresearch.com/sample/3421
Vaccine Contract Manufacturing Market Scope
| Report Coverage | Details |
| Growth Rate from 2023 to 2032 | CAGR of 11.2% |
| Market Size in 2023 | USD 3.43 Billion |
| Market Size by 2032 | USD 8.92 Billion |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segments Covered | By Vaccine Type, By Workflow, and By Application |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Read More: Tuberculosis Diagnostics Market Size To Grow USD 3.67 Billion by 2032
Vaccine Contract Manufacturing Market Players:
- Lonza
- FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
- Ajinomoto Althea, Inc.
- Merck KgaA
- Cytovance Biologics
- Catalent, Inc.
- IDT Biologika GmbH
- Albany Molecular Research, Inc.
- PRA Health Sciences
- ICON plc.
- Pharmaceutical Product Development, LLC
- Cobra Bio
- Paragon Bioservices, Inc.
Data Sources and Methodology
To gather comprehensive insights on the Global Vaccine contract manufacturing Market, we relied on a range of data sources and followed a well-defined methodology. Our approach involved interactions with industry experts and key stakeholders across the market’s value chain, including management organizations, processing organizations, and analytics service providers.
We followed a rigorous data analysis process to ensure the quality and credibility of our research. The gathered information was carefully evaluated, and relevant quantitative data was subjected to statistical analysis. By employing robust analytical techniques, we were able to derive meaningful insights and present a comprehensive overview of the Global Vaccine contract manufacturing Market.
The most resonating, simple, genuine, and important causes because of which you must decide to buy the Vaccine contract manufacturing market report exclusively from precedence research
- The research report has been meticulously crafted to provide comprehensive knowledge on essential marketing strategies and a holistic understanding of crucial marketing plans spanning the forecasted period from 2023 to 2032.
Key Features of the Report:
- Comprehensive Coverage: The report extensively encompasses a detailed explanation of highly effective analytical marketing methods applicable to companies across all industry sectors.
- Decision-Making Enhancement: It outlines a concise overview of the decision-making process while highlighting key techniques to enhance it, ensuring favorable business outcomes in the future.
- Articulated R&D Approach: The report presents a well-defined approach to conducting research and development (R&D) activities, enabling accurate data acquisition on current and future marketing conditions.
Market Segmentation:
By Vaccine Type
- Attenuated
- Inactivated
- Subunit-based
- Toxoid-based
- DNA-based
By Workflow
- Downstream
- Upstream
By Application
- Human Use
- Veterinary
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Reasons to Consider Purchasing the Report:
- Enhance your market research capabilities by accessing this comprehensive and precise report on the global Vaccine contract manufacturing market.
- Gain a thorough understanding of the overall market landscape and be prepared to overcome challenges while ensuring robust growth.
- Benefit from in-depth research and analysis of the latest trends shaping the global Vaccine contract manufacturing market.
- Obtain detailed insights into evolving market trends, current and future technologies, and strategic approaches employed by key players in the global Vaccine contract manufacturing market.
- Receive valuable recommendations and guidance for both new entrants and established players seeking further market expansion.
- Discover not only the cutting-edge technological advancements in the global Vaccine contract manufacturing market but also the strategic plans of industry leaders.
Table of Content
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology (Premium Insights)
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Vaccine Contract Manufacturing Market
5.1. COVID-19 Landscape: Vaccine Contract Manufacturing Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Vaccine Contract Manufacturing Market, By Vaccine Type
8.1. Vaccine Contract Manufacturing Market, by Vaccine Type, 2023-2032
8.1.1 Attenuated
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. Inactivated
8.1.2.1. Market Revenue and Forecast (2020-2032)
8.1.3. Subunit-based
8.1.3.1. Market Revenue and Forecast (2020-2032)
8.1.4. Toxoid-based
8.1.4.1. Market Revenue and Forecast (2020-2032)
8.1.5. DNA-based
8.1.5.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global Vaccine Contract Manufacturing Market, By Workflow
9.1. Vaccine Contract Manufacturing Market, by Workflow, 2023-2032
9.1.1. Downstream
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Upstream
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Electrolyte
9.1.3.1. Market Revenue and Forecast (2020-2032)
9.1.4. Separator
9.1.4.1. Market Revenue and Forecast (2020-2032)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global Vaccine Contract Manufacturing Market, By Application
10.1. Vaccine Contract Manufacturing Market, by Application, 2023-2032
10.1.1. Human Use
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Veterinary
10.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 11. Global Vaccine Contract Manufacturing Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.1.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.1.3. Market Revenue and Forecast, by Application (2020-2032)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.1.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.1.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.1.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.1.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.3. Market Revenue and Forecast, by Application (2020-2032)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.6.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.6.3. Market Revenue and Forecast, by Application (2020-2032)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.7.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.7.3. Market Revenue and Forecast, by Application (2020-2032)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.3. Market Revenue and Forecast, by Application (2020-2032)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.6.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.6.3. Market Revenue and Forecast, by Application (2020-2032)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.7.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.7.3. Market Revenue and Forecast, by Application (2020-2032)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.6.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.6.3. Market Revenue and Forecast, by Application (2020-2032)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.7.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.7.3. Market Revenue and Forecast, by Application (2020-2032)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.5.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.5.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.5.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.5.5.3. Market Revenue and Forecast, by Application (2020-2032)
Chapter 12. Company Profiles
12.1. Lonza
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Ajinomoto Althea, Inc.
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Merck KgaA
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Cytovance Biologics
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Catalent, Inc.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. IDT Biologika GmbH
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Albany Molecular Research, Inc.
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. PRA Health Sciences
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. ICON plc.
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
About Us:
Our team comprises a dedicated group of research analysts and management consultants who are driven by a unified vision: assisting individuals and organizations in realizing their strategic objectives, both immediate and long-term, through the provision of comprehensive research services. At Precedence Research, we have positioned ourselves to cater to the needs of a diverse range of entities, including established companies, startups, and non-profit organizations across various sectors. Our expertise extends to industries such as packaging, automotive, healthcare, chemicals and materials, industrial automation, consumer products, electronics and semiconductors, IT and telecommunications, and energy. With a wealth of experience within our ranks, our skilled analysts are equipped with extensive knowledge of the research landscape.
Contact Us
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
