The global vascular closure devices market size was valued at USD 1.75 billion in 2023 and is projected to reach around USD 3.30 billion by 2033, growing at a CAGR of 6.55% from 2024 to 2033.
Key Points
- North America led the market with the biggest market share of 36% in 2023.
- Asia Pacific is expected to witness notable growth in the market during the forecast period.
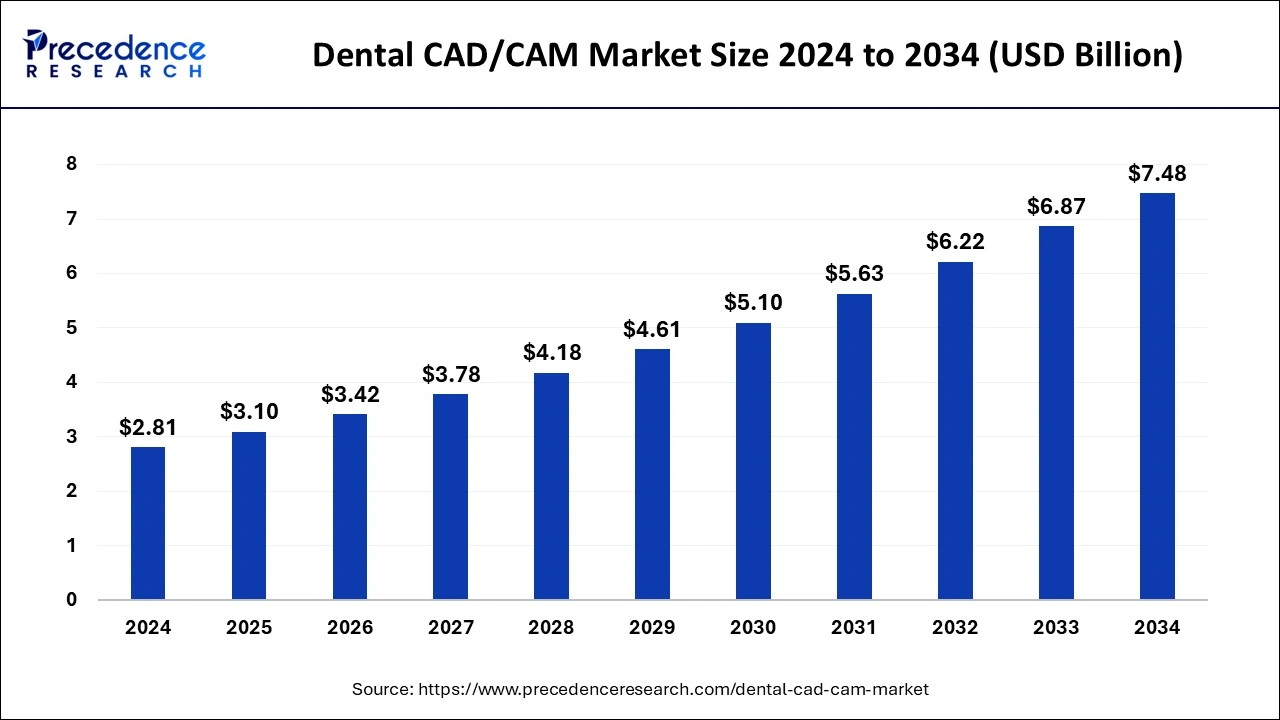
- By type, the in-lab system segment dominated the dental CAD/CAM market in 2023.
- By component, the hardware segment had the highest market share in 2023.
- By component, the software segment is expected to grow in the market during the forecast period.
- By end-user, the dental clinics segment dominated the market with the largest share in 2023.
The vascular closure devices market is witnessing significant growth attributed to the rising prevalence of cardiovascular diseases globally. Vascular closure devices are medical devices used to achieve hemostasis of the small artery puncture site following catheterization procedures. These devices are crucial in reducing the time required for post-procedural care, minimizing complications such as bleeding, and facilitating early ambulation of patients. The market for vascular closure devices is driven by advancements in minimally invasive procedures, increasing adoption of interventional cardiology techniques, and the growing geriatric population susceptible to cardiovascular ailments.
Get a Sample: https://www.precedenceresearch.com/sample/3946
Table of Contents
ToggleGrowth Factors
Several factors contribute to the growth of the vascular closure devices market. One of the primary growth drivers is the rising incidence of cardiovascular diseases, including coronary artery disease, peripheral artery disease, and heart failure. As the prevalence of these conditions continues to escalate globally, the demand for vascular closure devices for managing catheterization procedures also increases. Moreover, technological advancements in vascular closure devices, such as the development of next-generation hemostatic devices with enhanced safety and efficacy profiles, propel market growth. Additionally, the growing preference for minimally invasive procedures over traditional surgical interventions fosters the adoption of vascular closure devices, as they offer quicker recovery times and reduced hospital stays.
Vascular Closure Devices Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 6.55% |
| Global Market Size in 2023 | USD 1.75 Billion |
| Global Market Size by 2033 | USD 3.30 Billion |
| U.S. Market Size in 2023 | USD 530 Million |
| U.S. Market Size by 2033 | USD 990 Million |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Type, By Access, and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Vascular Closure Devices Market Dynamics
Drivers:
Several key drivers are fueling the expansion of the vascular closure devices market. One significant driver is the increasing adoption of catheter-based interventions for the diagnosis and treatment of cardiovascular diseases. Catheterization procedures, such as angiography, angioplasty, and stent placement, are commonly performed to visualize and treat blockages in blood vessels. Vascular closure devices play a crucial role in ensuring safe and effective hemostasis following these procedures, thereby driving their demand. Furthermore, the growing emphasis on improving healthcare infrastructure in emerging economies, coupled with rising healthcare expenditure, contributes to market growth. The introduction of innovative vascular closure devices with features such as rapid hemostasis, reduced complication rates, and compatibility with various access sites further accelerates market expansion.
Restraints:
Despite the favorable market conditions, several factors restrain the growth of the vascular closure devices market. One significant restraint is the high cost associated with advanced vascular closure devices. The initial investment required for purchasing these devices, along with the need for specialized training for healthcare professionals, can pose financial challenges, particularly in resource-limited settings. Moreover, concerns regarding the risk of complications associated with vascular closure devices, such as vascular complications, pseudoaneurysm formation, and device-related thrombosis, may deter their widespread adoption. Additionally, stringent regulatory requirements for product approval and the presence of alternative hemostasis techniques, such as manual compression and use of mechanical closure devices, present barriers to market growth.
Opportunity:
Despite the challenges, the vascular closure devices market presents lucrative opportunities for manufacturers and stakeholders. One key opportunity lies in the untapped potential of emerging markets, where there is a growing demand for advanced medical devices due to improving healthcare infrastructure and rising disposable incomes. Manufacturers can capitalize on this opportunity by expanding their presence in these regions and introducing cost-effective vascular closure solutions tailored to local needs. Furthermore, ongoing research and development activities aimed at enhancing the safety and efficacy of vascular closure devices present avenues for innovation and market expansion. The introduction of novel materials, advanced hemostatic mechanisms, and integrated closure systems holds promise for addressing existing unmet needs and driving adoption among healthcare providers.
Read Also: Tubeless Insulin Pump Market Size to Attain USD 11.93 Bn by 2033
Region Insights:
The vascular closure devices market exhibits regional variations influenced by factors such as disease prevalence, healthcare infrastructure, regulatory environment, and economic conditions. North America holds a prominent position in the market, attributed to the high incidence of cardiovascular diseases, well-established healthcare systems, and early adoption of advanced medical technologies. Europe also represents a significant market share, driven by favorable reimbursement policies, increasing geriatric population, and technological advancements in interventional cardiology. In the Asia Pacific region, rapid urbanization, changing lifestyle patterns, and improving access to healthcare services contribute to market growth. Moreover, initiatives aimed at promoting awareness about cardiovascular health and the adoption of minimally invasive procedures further fuel market expansion in this region. Other regions such as Latin America, the Middle East, and Africa offer growth opportunities supported by improving healthcare infrastructure and rising healthcare expenditure.
This comprehensive overview highlights the growth trajectory, drivers, restraints, opportunities, and regional insights shaping the vascular closure devices market. As the prevalence of cardiovascular diseases continues to rise and healthcare systems focus on improving patient outcomes, the demand for advanced vascular closure solutions is expected to witness sustained growth globally. Manufacturers and stakeholders in the healthcare industry must strategically position themselves to capitalize on emerging opportunities and address evolving market dynamics to ensure long-term success in the vascular closure devices market.
Recent Developments
- In February 2023, the LockeT product was introduced to the market by Catheter Precision, Inc., a wholly-owned subsidiary of Ra Medical Systems, Inc. The first shipments of the product to its distributors will start right away. When a catheter is inserted through the skin into a blood artery and subsequently removed after an operation, LockeT can be utilized in combination with the closure of the percutaneous wound site. LockeT is used to keep the sutures in place after the doctor has sutured the vessel and the location. The lockeT can be utilized in place of or in addition to closing devices. These devices are Angioseal, marketed by Terumo, Vascade, marketed by Cardiva, a division of Haemonetics, and Perclose, marketed by Abbott.
Vascular Closure Devices Market Companies
- Medtronic
- Abbott Vascular
- Biotronik GMBH & CO. KG
- COOK
- Merit Medical Systems, Inc.
- C. R. Bard, Inc.
- Boston Scientific Corporation
- ESSENTIAL MEDICAL, Inc.
- Cardinal Health
- W L. Gore & Associates
Segments Covered in the Report
By Type
- Active Vascular Closure Device
- Passive Vascular Closure Device
By Access
- Radial
- Femoral
By End-use
- Ambulatory Surgical Centers
- Hospitals
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.uswebwire.com/
